One ERP. Every Industry. Global Presence. Powered by Azaalea AI

One ERP. Every Industry. Global Presence. Powered by
Batch Manufacturing Practice
It ensures that every product leaving the line meets defined standards of quality, compliance, and consistency.
Batch Manufacturing Practice
It ensures that every product leaving the line meets defined standards of quality, compliance, and consistency.
Why choose this module
Unified control of formulations, ingredients, and process routes.
Real-time batch monitoring and automated weighing instructions.
End-to-end traceability of materials, batches, and products.
Predefined workflows for blending, mixing, and packaging operations.
Compliance support for GMP, FDA, ISO, and other standards.
Production Flow
One seamless process from formulation to fulfillment
Define product recipes and formulations with version control.
Allocate materials and schedule batches based on demand.
Capture batch execution data automatically from production lines.
Conduct in-process and post-production quality inspections.
Generate batch genealogy for full backward and forward traceability.
Compare actual yield against planned output to improve accuracy.
Record batch-wise equipment usage and maintenance logs.
Approve batches for dispatch after compliance verification.
Core capabilities
- Define ingredient ratios and process parameters.
- Manage multiple versions of formulations.
- Control tolerance levels and production stages.
- Store digital SOPs for each process step.
- Maintain audit trail of all recipe modifications.
- Automate production scheduling and resource allocation.
- Record batch start, hold, and completion events.
- Generate process sheets and barcode labels.
- Real-time tracking of batch performance.
- Prevent batch duplication or material mismatch.
- Link each batch with mandatory quality tests.
- Capture lab results and approvals electronically.
- Integrate non-conformance and corrective actions.
- Maintain batch-wise certificates of analysis.
- Monitor quality trends and deviations.
- Maintain full genealogy of materials and finished goods.
- Enable forward and backward tracking in seconds.
- Auto-generate compliance and audit reports.
- Follow GMP and ISO protocols digitally.
- Provide digital signatures and document timestamps.
- Compare planned vs actual cost per batch.
- Track wastage, rework, and yield deviation.
- Integrate with finance for cost accounting.
- Identify low-performing batches automatically.
- Optimize formulation cost with historical insights.
- Interactive dashboards for production KPIs.
- Monitor equipment utilization and downtime.
- Forecast material requirements based on demand.
- Visualize production trends and efficiency metrics.
- Custom reports for regulatory audits.
Integrations & APIs
- Connect sensors for temperature, pressure, and mixing data.
- Automate equipment log entries for compliance tracking.
- Sync data between Quality, Inventory, and Finance modules.
- Enable real-time production insights for managers.
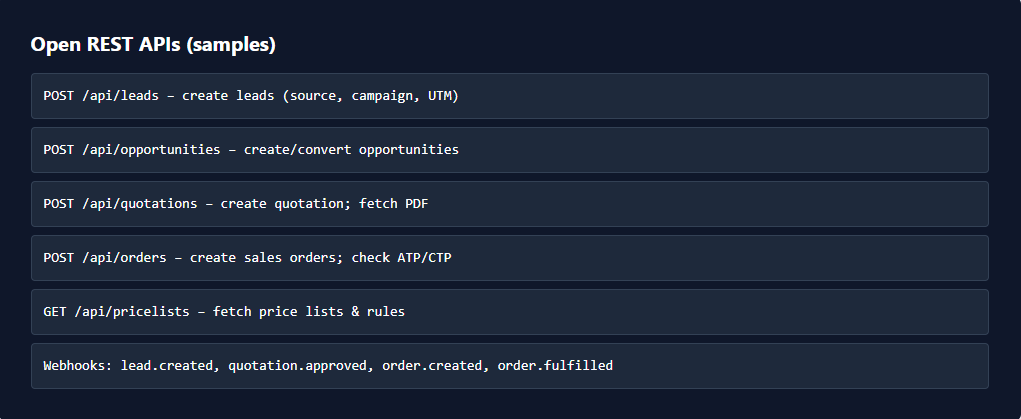
Automation examples (no-code)
Auto-approve batches when quality test passes.
Notify supervisors when yield variance exceeds limits.
Generate batch certificates post-production.
Route failed batches to reprocessing automatically.
Role-based views
Production Manager
Monitor batch status, yield, and work order progress.
Quality Inspector
Access lab test results, compliance data, and hold/release approvals.
Store Executive
View material issues, shortages, and reorders linked to batches.
Finance Controller
Track cost per batch and record asset consumption.
Maintenance Head
Check machine utilization and maintenance schedules linked with batches.
KPIs to track
Batch cycle time
Yield variance %
First-pass quality rate
Batch cost variance
Downtime per batch
Rework frequency
Frequently
Asked Questions
Get answers to common questions about Batch Manufacturing Practice

What industries can use Batch Manufacturing Practice in eresource ERP?
Batch Manufacturing Practice is ideal for process-based sectors like pharmaceuticals, chemicals, food, cosmetics, and paint manufacturing.
Can the system handle multiple batch versions and formula changes?
Yes, eresource ERP maintains full version control for formulations, recipes, and batch parameters.
How does Batch Manufacturing Practice ensure product traceability?
It links raw materials, production data, and dispatch details through batch IDs for complete traceability.
Does it comply with global quality standards?
Yes, eresource ERP supports GMP, ISO, and FDA compliance with digital documentation.
Can Batch Manufacturing Practice integrate with IoT devices or lab systems?
It supports integration with IoT sensors and LIMS for real-time monitoring and reporting.
Is Batch Manufacturing Practice scalable for multi-location production plants?
Absolutely. The system supports centralized control with plant-wise batch tracking.
Consistency in Every Batch
With eresource ERP’s Batch Manufacturing Practice, you gain unified control over formulations, processes, and quality—ensuring every batch meets your brand promise with precision and compliance.
Email: sales@eresourceerp.com · Website: www.eresourceerp.com
